Chemical Database
2,4,6-trinitrophenol
Identifications
- Formula: (NO2)3C6H2OH
- Formula: C6H2KN3O7
- Formula: C6H3N3O7
- Formula: NH4C6H3O7
- CAS Number: 131-74-8
- CAS Number: 146-84-9
- CAS Number: 189195-39-9
- CAS Number: 190402-10-9
- CAS Number: 26488-89-1
- CAS Number: 29663-11-4
- CAS Number: 3324-58-1
- CAS Number: 573-83-1
- CAS Number: 6477-64-1
- CAS Number: 68131-74-8
- CAS Number: 88-89-1
- CCOHS Record Number: 515
- RCRA Waste Number: P009
- RTECS Number: TJ7875000
- Synonyms/Related:
- 1,3,5-Trinitrophenol
- 10015990
- 2, 4,6-Trinitrofenolo
- 2,4,6-Trinitrofenol
- 2,4,6-Trinitrofenol [Dutch]
- 2,4,6-Trinitrofenolo
- 2,4,6-Trinitrofenolo [Italian]
- 2,4,6-Trinitrophenol
- 2,4,6-Trinitrophenol ammonium salt
- 2,4,6-Trinitrophenol, sodium salt
- 2,4,6-Trinitrophenyl
- 2-Hydroxy-1,3,5-trinitrobenzene
- 21939.3
- 28108.3
- 28109.3
- Acide picrique
- Acide picrique [French]
- Acido picrico
- Acido picrico [Italian]
- Acidum picrinicum
- Ammonium carbazoate
- Ammonium Picrate
- Ammonium picrate, dry or wetted with <10% water, by mass [UN0004] [Explosive 1.1D]
- Ammonium picrate, dry or wetted with less than 10 percent water, by mass
- Ammonium picrate, wetted with not <10% water, by mass [UN1310] [Flammable solid]
- Ammonium picrate, wetted with not less than 10 percent water, by mass
- Ammonium picronitrate
- Ash
- c0945
- Carbazotic Acid
- CI 10305
- Explosive D
- Hager's reagent
- Kyselina pikrova
- Kyselina pikrova [Czech]
- Lead dipicrate
- Lyddite
- Melinite
- NA1344
- NISTC88891
- Nitroxanthic acid
- Obeline picrate
- Pertite
- Phenol trinitrate
- phenol, 2,4,6-trinitro
- Phenol, 2,4,6-trinitro-
- Phenol, 2,4,6-trinitro-, ammonium salt
- Phenol, 2,4,6-trinitro-, potassium salt
- Phenol, 2,4,6-trinitro-, silver (1+) salt
- Phenol, 2,4,6-trinitro-, silver(1+) salt (9CI)
- Phenol, 2,4,6-trinitro-, sodium salt
- Phenoltrinitrate
- Picragol
- Picral
- Picratol
- Picric acid
- Picric acid (dry)
- Picric acid (wet)
- Picric acid sodium salt
- Picric acid, ammonium salt
- Picric acid, dry
- Picric acid, dry or wetted with < 30% water, by mass
- Picric acid, dry or wetted with less than 30 percent water, by mass
- Picric acid, potassium salt
- Picric acid, potassium salt (8CI)
- Picric acid, silver (1+) salt
- Picric acid, silver(1+) salt (8CI)
- Picric acid, sodium derivative
- Picric acid, sodium salt
- Picric acid, sodium salt (8CI)
- Picric acid, wet
- Picric acid, wet, with not <10% water [NA1344] [Flammable solid]
- Picric acid, wet, with not <10% water [NA1344] [Flammable solid]
- Picric acid, wet, with not less than 10% water
- Picric acid, wetted with not less than 10% water
- Picronitric acid
- Picrotol
- Pikrinezuur
- Pikrinezuur [Dutch]
- Pikrinsaeure
- Pikrinsaeure [German]
- Pikrynowy kwas
- Pikrynowy kwas [Polish]
- Potassium Picrate
- Potassium trinitrophenolate
- Reflorit
- Shimose
- Silver picrate
- Silver picrate (dry) [Forbidden]
- Silver picrate, wetted with not <30% water, by mass [UN1347] [Flammable solid]
- Silver picrate, wetted with not less than 30% water
- Silver, (picryloxy)-
- Sodium 2,4, 6-trinitrophenolate
- Sodium 2,4,6-trinitrophenolate
- Sodium picrate
- Sodium trinitrophenolate
- Sodium, (picryloxy)-
- TNF
- Trinitrophenol
- Trinitrophenol (picric acid) , wetted, with not less than 10 percent water by mass
- Trinitrophenol or picric acid, dry or wetted with <30% water, by mass [UN0154] [Explosive 1.1D]
- Trinitrophenol or picric acid, dry or wetted with <30% water, by mass [UN0154] [Explosive 1.1D]
- Trinitrophenol, dry or wetted with < 30% water, by mass
- Trinitrophenol, wetted with not <30% water, by mass [UN1344] [Flammable solid]
- Trinitrophenol, wetted with not <30% water, by mass [UN1344] [Flammable solid]
- Trinitrophenol, wetted with not less than 10% water
- Trinitrophenol, wetted with not less than 30 percent water, by mass
- Trinitrophenol, wetted with not less than 30% water
Properties
- Boiling Point: K °C °F
- Flammability:
- Explosive Limits:
- Lower Explosive Limit: 0%
- Upper Explosive Limit: 0%
- Flammable Limits:
- Lower Flammable Limit: 0%
- Upper Flammable Limit: 0%
- Flash Point K °C °F
- Autoignition Temperature: K °C °F
- Explosive Limits:
- Incompatiblities:
- ammonia
- concrete
- copper
- corrosive to metals
- lead
- many metals
- plaster
- salts
- zinc
- Melting Point: K °C °F
Health & Regulatory Guidelines
- NFPA 704 Rating:
- Health Hazardard Rating: 3
- Fire Hazardard Rating: 4
- Reactivity Hazardard Rating: 4
- NIOSH Guidelines:
- OSHA Regulations:
- Health Risks: Toxic
- Notes: Corrosive to metals. An explosive mixture results when the aqueous solution crystallizes.
49 CFR 172.101 - Hazardous Materials Table
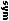 | Hazardous materials description and proper shipping names | Haz class or div | ID# | PG | LC | Spec prov §172.102 | Pack §173 | Qty limit | Vessel | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excep | Non Bulk | Bulk | Pass air/rail | Cargo air only | Loc | Other | |||||||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8A) | (8B) | (8C) | (9A) | (9B) | (10A) | (10B) |
| Current as of Oct. 6, 2011 | |||||||||||||
| Ammonium picrate, dry or wetted with less than 10 percent water, by mass | 1.1D | UN0004 | II | 1.1D | None | 62 | None | Forbid | Forbid | 10 | 5E, 19E | ||
| Ammonium picrate, wetted with not less than 10 percent water, by mass | 4.1 | UN1310 | I | 4.1 | 23, A2, N41 | None | 211 | None | Forbid | Forbid | D | 28, 36 | |
| Picric acid, see Trinitrophenol, etc | Forbid | Forbid | |||||||||||
2008 Emergency Response Guidebook Information
Produced by the US DOT the ERG is designed to aid first responders in quickly identifying specific or generic hazards of materials involved in an incident and protecting themselves and the general public during the initial response phase of an incident.
| UN# | Guide | Name of Material | ISO | H20 React | TIH Gas(es) |
|---|---|---|---|---|---|
| Current as of: Oct. 2, 2011 | |||||
| 1344 | 113 | Picric acid, wet, with not less than 10% water | No | No | |
| 1344 | 113 | Trinitrophenol, wetted with not less than 30% water | No | No | |
| 1347 | 113 | Silver picrate, wetted with not less than 30% water | No | No | |
| 3364 | 113 | Picric acid, wetted with not less than 10% water | No | No | |
| 3364 | 113 | Trinitrophenol, wetted with not less than 10% water | No | No | |
Related Resources
- USDOT Hazardous Materials Table 49 CFR 172.101An online version of the USDOT's listing of hazardous materials from 49CFR 172.101. This table can be sorted by proper shipping name, UN/NA ID and/or by primary hazard class/division.
- 2008 ERG (Emergency Response Guidebook)Have you ever wondered what those four digit numbers on the placards on the side of trucks and rail cars mean? Our online 2008 ERG will give you your answer. This is an online version of the guidebook produced by the USDOT for first responders during the initial phase of a Dangerous goods/HazMat incident. ERG data last verified/updated Oct. 2, 2011
- US DOT Hazardous Materials Transportation PlacardsHazardous materials placards (DOT placards) are required when shipping hazardous materials in the United States, Canada and Mexico. These pages provide US DOT definitions for each hazmat placard.
- Guide for Handling Household ChemicalsThings you can do to make your home safer.
- Molarity, Molality and NormalityIntroduces stoichiometry and explains the differences between molarity, molality and normality.
- Molar Mass Calculations and Javascript CalculatorMolar mass calculations are explained and there is a JavaScript calculator to aid calculations.
- Periodic Table of ElementsProvides comprehensive data for each element of the periodic table of elements including up to 40 properties, names in 10 languages and common chemical compounds. Information also provided for 3,600 nuclides and 4,400 nuclide decay modes.
Editor's note: Some chemicals in this database contain more information than others due to the original reason this information was collected and how the compilation was accomplished.
While working with material safety data sheets (MSDS), I found that manufacturers sometimes used obscure names for constituent chemicals and I didn't always have a good idea of what I was dealing with. To resolve this problem, over the years, I compiled chemical names and identifiers into a personal database, cross referencing regulatory and health safety information when possible. Colleagues and friends eventually started suggesting that I make my data available on this website so that others could benefit from my efforts -- which I finally did in 2004. The more common, regulated and/or hazardous a chemical is, the more information I will have likely collected it.
Trademarks
If you are aware of any synonyms listed above that are registered trademarks, please contact us with relevant information so that trademarks can be appropriately noted.
Notes about mixtures
Some chemicals listed in this database or not pure chemical compounds, rather they are mixtures/solutions of chemicals. It is not uncommon for wide range of molar ratios of a mixture to be lumped together as "synonyms" of the same "chemical". In some instances chemicals that are very similar from a health & safety and/or regulatory standpoint also may have been lumped together.
Reference Sources
Data for this database was compiled from: hundreds of Material Safety Data Sheets (MSDS) of common industrial and household products; the Hazardous Materials Table from the United States "Code of Federal Regulations" title 49 section 172.101; the National Institute for Occupational Safety and Health Pocket Guide to Chemical Hazards; the US DOT 1996, 2000 & 2004 Emergency Response Guidebooks; U.S. National Library of Medicine and many other related resources.
Disclaimer
WARNING: These pages are for general reference and educational purposes only and MUST NOT be relied upon as a sole source to determine regulatory compliance or where matters of life and health are concerned. This site and the author do not warrant or guarantee the accuracy or the sufficiency of the information provided and do not assume any responsibility for its use.
To ensure regulatory compliance when transporting hazardous materials or dangerous goods, one must receive proper training and certification from a qualified instructor and refer to the current year's Code of Federal Regulations Title 49 (49CFR) or your country's shipping regulations. In matters regarding workplace safety, refer to current OSHA regulations (29CFR) and NIOSH guidelines or your own country's health and safety regulations. No one should ever enter into a hazardous environment without proper training from qualified instructors.
Citing this page
If you need to cite this page, you can copy this text:
Kenneth Barbalace. Chemical Database - 2,4,6-trinitrophenol. EnvironmentalChemistry.com. 1995 - 2026. Accessed on-line: 2/21/2026
https://EnvironmentalChemistry.com/yogi/chemicals/cn/2%2C4%2C6-trinitrophenol.html
.
Linking to this page
If you would like to link to this page from your website, blog, etc., copy and paste this link code (in red) and modify it to suit your needs:
<a href="https://EnvironmentalChemistry.com/yogi/chemicals/cn/2%2C4%2C6-trinitrophenol.html">echo Chemical Database: 2,4,6-trinitrophenol (EnvironmentalChemistry.com)</a>- This page contains information on the chemical 2,4,6-trinitrophenol including: 126 synonyms/identifiers; U.S. Code of Federal Regulations Title 49 Section 172 shipping regulations and 3 proper shipping names; USDOT 2008 Emergency Response Guidebook initial response information for 5 related materials.
.